|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Monographs High School High School Chemistry Chemistry Matter Matter  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Created: 27 Jun, 2010; Last Modified: 10 Aug, 2021 The page has been upgraded to a new home. Please follow this link. Matter - 03Laws of Chemical Combination
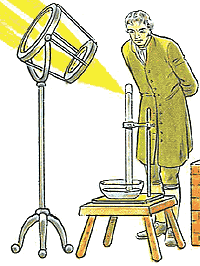
It was the Englishman Robert Boyle (17th century) who, through research on the behaviour of gases, provided clear evidence for the atomic makeup of matter. He was the first to define an element as a substance that cannot be chemically broken down further. He believed that a number of different elements might exist in nature. Law of Conservation of MassIn 1774, Joseph Priestley isolated the gas oxygen by heating mercuric oxide. Soon thereafter, Antoine Lavoisier claimed that oxygen is the key substance involved in combustion (burning). He also demonstrated that when combustion is carried out in a closed container, the mass of the final products of combustion exactly equals the mass of the starting reactants. This led to the statement of the Law of Conservation of Mass: Law of Conservation of Mass
Mass is neither created nor destroyed in chemical reactions.
In an experiment, Science today knows that matter can be converted into energy (and vice-versa). Hence, during all chemical and physical changes, the total mass+energy before the change is equal to the total mass+energy after the change. Still, as there is no detectable change in mass in an ordinary chemical reaction, the law of conservation of mass is still valid. Silicon dioxide, made up of elements silicon and oxygen, contains
Law of Definite Proportions / Constant CompositionIn the years following Lavoisier, the French chemist Joseph Proust formulated a second fundamental law of chemical science – the Law of Definite Proportions.
Law of Definite Proportions (Law of Constant Composition)
In a given compound, the constituent elements are always combined in the same proportions by mass, regardless of the origin or mode of preparation of the compound.What this law means is that when elements react chemically, they combine in specific proportions, not in random proportions. A sample of pure water, whatever the source, always contains The compound cupric oxide may be prepared by any one of the following methods –
Experiment 1: Mass of mercuric oxide Experiment 2: Mass of mercuric oxide In both cases, the mercury to oxygen ratio is the same, thus conforming to the law of definite proportions. Dalton’s Atomic Theory of MatterRun up to the theory
The explanation for the law of conservation of mass and the law of definite proportions was provided by the English schoolteacher John Dalton in 1808. Dalton reasoned as follows: (McMurray & Fay, Chemistry 4ed, p. 40)
Dalton's Atomic TheoryThus, Dalton’s Atomic Theory can be stated as follows:
Drawbacks of Dalton's theoryIn light of the current state of knowledge in the field of Chemistry, Dalton’s theory had a few drawbacks. According to Dalton’s postulates,
Importance of Dalton's theoryNotwithstanding these drawbacks, the importance of Dalton’s theory should not be underestimated. He displayed exceptional insight into the nature of matter. His ideas provided a framework that could be modified and expanded by later scientists. Thus John Dalton is often considered to be the father of modern atomic theory. Feedback
If your email client is configured, click here to send your feedback.
Otherwise, send an email to feedback@mentorials.com with subject line: "Feedback: Laws of Chemical Combination & Dalton's Atomic Theory". List of ReferencesBrent, R, The Golden Book of Chemistry Experiments, NY, USA: Golden Press, 1960. McMurray, J & Fay, RC, Chemistry, 4th edn, USA: Prentice Hall, 2003. BibliographyMahan, BM & Meyers, RJ, University Chemistry, 4th edn, New Delhi: Addison Wesley Longman, 1987. McMurray, J & Fay, RC, Chemistry, 4th edn, USA: Prentice Hall, 2003. Whitten, KW, Davis, RE, Peck, L & Stanley, GG, General Chemistry, 7th edn, Belmont, USA: Thomson Brooks/Cole, 2004.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Monographs High School High School Chemistry Chemistry Matter Matter  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Copyright © 2009-2025 Mentorials.com. All Rights Reserved.
[Site best viewed at 1024 x 768 resolution, using Mozilla Firefox 3.5 and above] Some clipart images used herein were obtained from IMSI's MasteClips® and MasterPhotos™ Premium Image Collection. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 High School
High School Mathematics
Mathematics